Show Advanced KnowledgeHide Advanced KnowledgeAmino acids
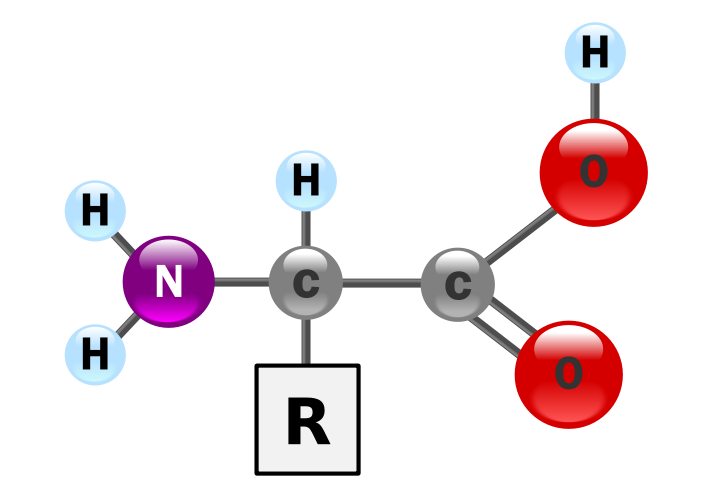
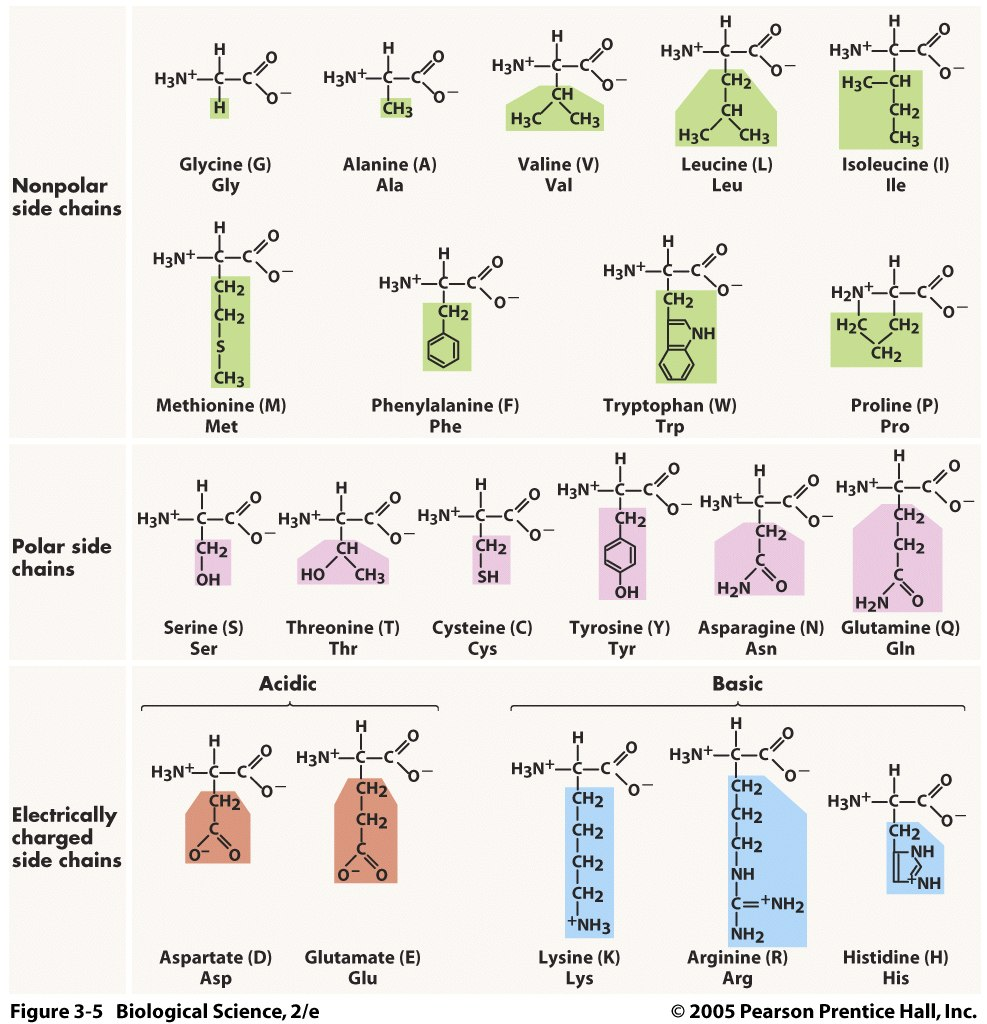
Amino acids are composed of amine (-NH2) and carboxylic acid (-COOH) groups (see Figure 1D.1). A side-chain R largly determines its physicochemical properties are specific to each amino acid (See Figure 1D.2).
While more than 500 amino acids exist we are mainly interested in proteinogenic amino acids - the amino acids that are cotranslationally combined to form proteins. Of the 23 proteinogenic amino acids only approx. 20 are encoded by eukaryotic genes.
Detail: The standard genetic code encodes for 20 amino acids. Using a mechanism called translational recoding a stop codon be give rise to the 21th amino acid selenocysteine.
Of these 20 amino acids, humans can synthesize 11 amino acids. The remaining essential amino acids must be supplied in the diet.
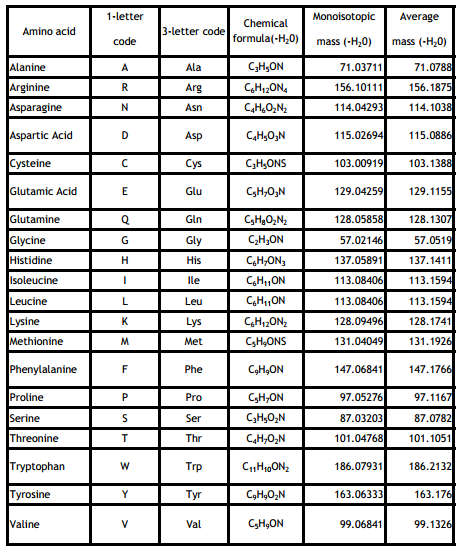
Amino acid mass tables in mass spectrometry usually contain the amino acids in dehydrated (loss of water; -H2O) form. This is mainly done for easier mass calculations as we will see in the section on peptide bond formation.
Exercise: What is the nominal mass of serine (without dehydration!)?
Leu and Ile (L/I) are structural isomers and therefor have the same mass. Molecules that have the same mass are called isobaric. Isobaric molecules can't be distinguised by mass alone.