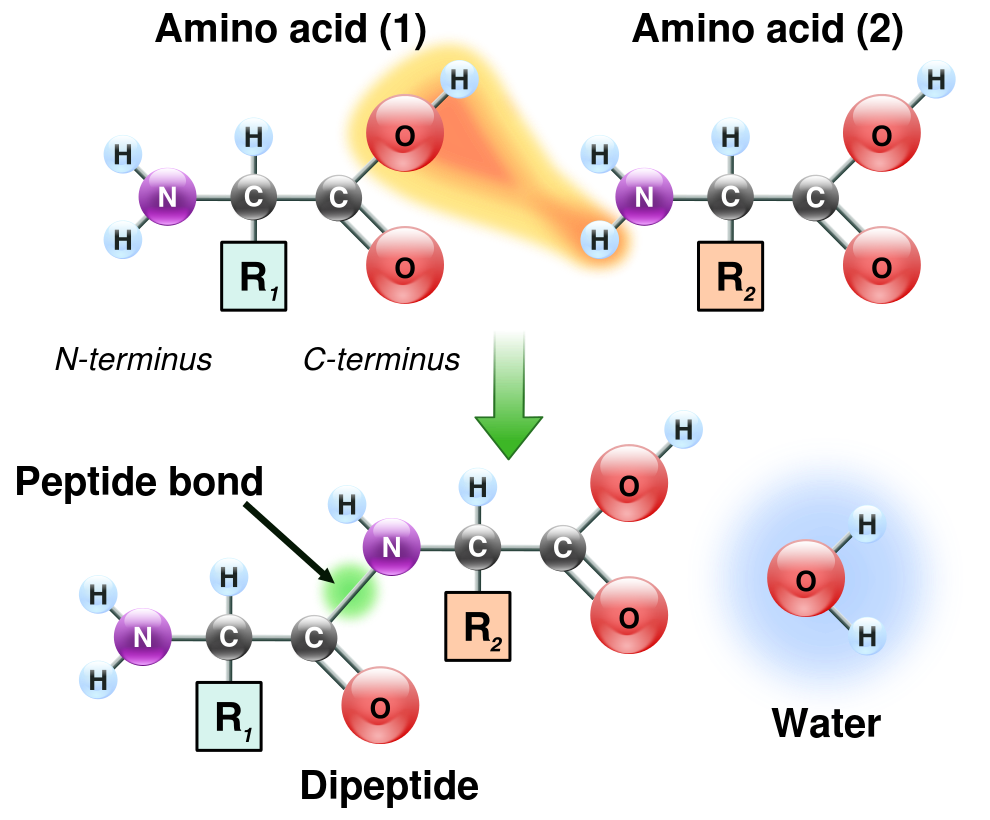
Show Advanced KnowledgeHide Advanced KnowledgePeptide bond formation
Amino acids are building blocks of proteins. They can be (under certain constraints) freely combined to form longer chains giving rise to an exponentially space of possible amino acids chains.
Not suprisingly, the capability to form longer chains is one of the main contributors to the complexity of the proteome.
Amino acid chains are formed in a condensation reaction that creates a novel peptide bond while one water molecule is set free (see Figure 1D.2).
To calculate the mass of a peptide we simply sum up the residue (=dehydrated amino acid) masses and add the mass of one water molecule. Easy isn’t it? This is because the mass table already contains the masses without the water molecule that is lost in the reaction. Calculating the mass from the original, non-dehydrated amino acid masses isn’t much more difficult. One just has to subtract all water molecules that are lost in each peptide bond formations from the sum of all amino acid masses.